Process technology machines and machine line parts in pharmaceutical production or food manufacturing, including those from Lödige Process Technology, are known to have to meet the highest hygienic standards. But how can these be implemented in practice? In other words: What are the potential risks and how can they be systematically avoided? Answers are provided by the comprehensive approach of hygienic design, which takes into account construction details as well as production peripherals and employee behaviour.
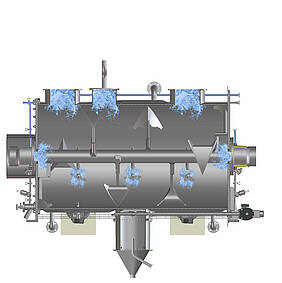
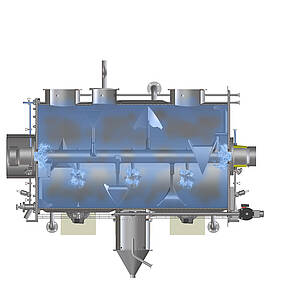
The example of mixing shows what hygienic design in process technology means in concrete terms: Mixing is one of the elementary processes in the process industry. Mixers guarantee the quality of food products as well as the consistent dosage of ingredients or pharmaceutically active components. An important aspect of hygienic design is therefore the cleanable layout of the mixer, including all parts coming in contact with the product. After all, the only way to reliably rule out contamination by microbes and unwanted particles in the end product as well as cross-contamination during product changes is if cleaning truly leaves no residues. Last but not least, this considerably increases the shelf life of perishable products in food or pharmaceutical production. Depending on which substances are processed, however, the protection of personnel and the environment can also play a role. Particularly in the production of active pharmaceutical ingredients, but also in sectors such as the chemical industry, employees must be protected, for instance, from contact with intermediate and end products, which can be harmful to health if used incorrectly or in excessive doses.
Concrete specifications for hygienic design
In any case, two factors have a decisive influence on the suitability for production processes with high hygiene requirements: the choice of suitable materials and the geometric design of the operating equipment. Experienced suppliers of mixers and other production equipment who, like Lödige, have the necessary process engineering know-how as well as the required expertise in hygienic design, develop their machines and machine lines according to clear criteria.
These include, for example, the following points:
• Material used
• Material combinations
• Geometry
• Connection technology
• Constructive details
• Components used
• Production engineering
• Surfaces and coatings
All these aspects are examined with regard to their suitability for use in hygienic production environments – weld seams, for example. In the mixing chamber, they must have the highest possible surface finish to prevent substances from accumulating during mixing. The same applies to the designs of inner angles and corners: Horizontal surfaces and right angles can lead to the accumulation of product residues, and thus to potential contamination. Machine components such as the seals for doors and flaps, cable ducts, feed-throughs and pipe connection types are also available in low-dead-space, hygienic designs. Another criterion that requires expertise is cleanability. Whether washing-in-place, cleaning-in-place or sterilisation-in-place: Hygienic execution is only guaranteed if all points in the machine can be reached and wetted by cleaning agents and disinfectants without obstruction.
Hygiene in production peripherals and employee behaviour
Hygienic design does not only concern the primary process areas such as the mixing chamber. The immediate surroundings of the process equipment must also be designed to be free of dead space and easy to clean. In addition to the aspects mentioned at the beginning, such as the design of angles, weld seams and surfaces, black/white separation is an important factor: It can prevent the spread of contamination. In technical terms, this means, for example, spatially separating the motor for driving a mixer or other process engineering machine from the machine itself. When applied to employees, the black and white principle means that pathogens are prevented from entering production by equipping changing rooms with decontamination facilities. The external and peripheral surfaces of machines and machine lines must also be considered in terms of hygienic design. For example, materials that easily become statically charged should be avoided to counteract the build-up of dust and product residues. The surface roughness is also decisive for possible deposits. According to EHEDG recommendations, it should be less than 0.8 µm. This ensures that even micro-organisms that have not been completely killed despite contact with disinfectants are reliably washed off the surface during cleaning. In addition to following procedures such as decontamination before commencing work, employees support hygienic production through compliant behaviour. This includes, for example, user administration, with the help of which all user activities (such as logging in or changing production settings) can be traced to a specific person at any time.
Institutes and standards guarantee safety
Suitability is guaranteed by guidelines from independent institutes, which contain clear recommendations, concept and design specifications.
These include, for example:
• FDA (U.S. Food and Drug Administration)
• EHEDG (European Hygienic Engineering and Design Group)
• ISPE (International Society for Pharmaceutical Engineering)
• ISO (International Organization for Standardization)
• DIN (Deutsches Institut für Normung (German Institute for Standardisation)
• GMP (Good Manufacturing Practice)
• 3A Sanitary Standards
• HACCP (Hazard Analysis and Critical Control Points)